The Importance of the Bioburden Testing for Medical Devices
702 Views
While an item fresh off the production line may be thought to be sterile, it can actually come into contact with numerous contaminants. To ensure that the final product is safe for use, a number of proper sanitation steps must be followed. One such step, particularly critical in the medical device manufacturing industry, is bioburden testing. Below is a brief description of what this assessment involves and why it is a key component in the sterilization process.
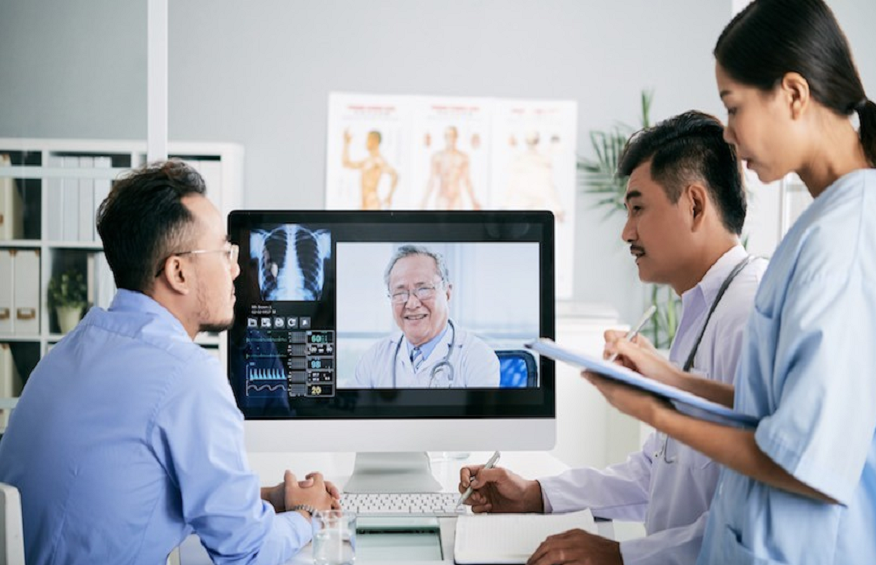
This infographic was created by Technical Safety Services, provider of cleanroom testing and certification